Post-operative bowel dysfunction is reported in 50-90% of patients after surgery to remove the rectum, and this results in an impaired quality of life in 30-50% of patients. The precise symptoms that patients report vary widely and include incontinence, constipation, an uncontrollable urge to rush to the toilet and a need to make multiple trips to the toilet in a short space of time. The wide variation in symptoms has made it difficult for experts to agree on which symptoms we should ask patients about. However recently a Danish group produced a patient-reported questionnaire that seems to be both simple to complete and thorough enough to addresses the key symptoms. The group called this tool the Low Anterior Resection Syndrome (LARS) score.
The LARS score was developed for a Danish population, therefore we need to translate the questionnaire into English (using a specific standardised process of forwards-backwards translation) and test it in a British population. This validation exercise will take the form of a cross-sectional study. We will send questionnaires to patients who were treated for rectal cancer with an anterior resection (removal of the rectum and then joining the bowel back together).
As well as validating the LARS questionnaire we also hope to get a better understanding of the risk factors for post-operative bowel dysfunction and we hope to be able to predict the group who will be at highest risk of developing severe symptoms.
Mr Brendan Moran, a Colorectal Consultant in Basingstoke, leads the study and The Pelican Cancer Foundation has funded it.
The study has now been completed and we validated the LARS questionnaire in 463 patients. The paper has been accepted by colorectal disease and the questionnaire is now being used in interventional studies to see how we can improve patient symptoms. We have also produced a simple consent aid that will be published shortly.
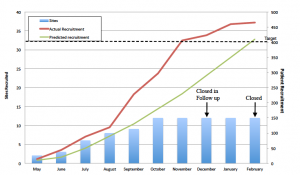
This study has now closed and results are available at:
Juul T, Battersby NJ, Christensen P, Janjua AZ, Branagan G, Laurberg S, Emmertsen KJ, Moran B; UK LARS Study Group. Validation of the English translation of the low anterior resection syndrome score. Colorectal Dis. 2015 Oct;17(10):908-16.
Battersby NJ, Juul T, Christensen P, Janjua AZ, Branagan G, Emmertsen KJ, Norton C, Hughes R, Laurberg S, Moran BJ; United Kingdom Low Anterior Resection Syndrome Study Group. Predicting the Risk of Bowel-Related Quality-of-Life Impairment After Restorative Resection for Rectal Cancer: A Multicenter Cross-Sectional Study. Dis Colon Rectum. 2016 Apr;59(4):270-80.
For further information please contact Mr Nick Battersby.
