Pelican no longer funds research into prostate cancer.
 Men with localised prostate cancer are forced to choose between two extremes of care – active surveillance or radical therapy.
Men with localised prostate cancer are forced to choose between two extremes of care – active surveillance or radical therapy.
The best evidence we have shows that the difference between these two very distinct approaches is not that substantial in terms of preventing an individual from dying of prostate cancer within a 10 year period – 14% mortality for active surveillance compared to 9% for men who had radical prostatectomy. On the other hand, we know that the side effects of radical treatments can be considerable (incontinence and impotence).
The profile and probability of these harmful outcomes depend to a large extent on the type of radical treatment, but all occur as a consequence of damaging tissue or structures that exist outside the prostate gland – the rhabdo-sphincter (part of the external urethral sphincter), the neurovascular bundles (nerves) and the rectal mucosa, respectively.
Refinements in the traditional radical therapies (various types of radiation therapy, including conformal or intensity modulated, or surgery to remove the prostate, either laparoscopic (keyhole) or robotic) have had little impact on the key treatment-related morbidities. The reason for this is that despite the refinements in the treatment their essential premise remains the same. The premise being that the whole gland is treated irrespective of the volume or position of the tumour.
Our research projects challenge the prevailing assumption that all men need to have their whole gland and the surrounding structures treated, irrespective of the volume and location of their prostate cancer. Therefore we have focused on supporting studies which involve focal therapy. Focal therapy is a form of treatment that ablates only parts of the prostate considered affected by clinically significant cancer. Focal therapy ablates discrete areas of known cancer in the prostate and can offer men a treatment option to control disease while preserving maximal amounts of healthy tissue.
Professor Mark Emberton and the urologists and radiologists at University College London Hospital (UCLH) are world leaders in different focal therapy treatments for prostate cancer. Mark Emberton’s team, in collaboration with Dr Clare Allen (Consultant Radiologist), Dr Alex Kirkham (Consultant in Radiology), Mr Hashim Ahmed, Mr Manit Arya and Mrs Caroline Moore (Consultant Urologist) are working to define the use of MRI scans in diagnosis and post-treatment follow-up of prostate cancer.
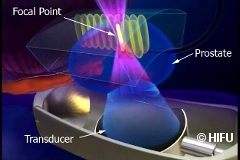
Pelican has supported four clinical trials at UCLH using High Intensity Ultrasound (HIFU) as a method of focal therapy.
- Hemi ablation (treating half the gland).
- Focal ablation (only treating lesions in one half of the gland).
- Index ablation (treating the largest tumour).
- FORECAST (2013 – treating recurrent disease)
Hemi-ablation HIFU: This technique destroys just half the gland and leaves the other half alone. This study aimed to determine whether in men who have prostate cancer confined to just one side of the gland, if treatment is limited to that side of the gland, is the treatment associated with acceptable levels of cancer control and a low rate of adverse side-effects? This trial is now complete and the outcomes were in 2011: ‘Focal Therapy for Localized Prostate Cancer: A Phase I/II Trial’ was published in the Journal of Urology– read the abstract from the Journal of Urology.
Focal-ablation HIFU: This technique destroys only the areas of the prostate that have tumours. This study aimed to determine whether in men who have prostate cancer confined to just one or even two discrete areas, if treatment is limited to just those two areas, is the treatment associated with acceptable levels of cancer control with a low rate of adverse side-effects? The outcomes have been very positive in terms of successful urinary and sexual function after treatment – read the abstract from NBCI PubMed.
Index-ablation HIFU: This technique targets only the ‘index’ lesion, the largest tumour – therefore an even smaller area of the gland is touched. Pelican supports the multicentre INDEX trial, which has developed from the results of the Focal Therapy concept. The primary outcomes of the trial look promising and the second phase of the study is in progress – read the abstract from NBCI PubMed.
As well as treating primary prostate cancer cases, the team also perform ‘salvage’ HIFU for those men who have failed other forms of treatment such as radiotherapy, brachytherapy and sometimes failed surgery if the recurrence is visible – an abstract of salvage HIFU results can be found on the NCBI PubMed site.
In 2013 Pelican also granted funds to Emberton and Ahmed to look at how side-effects of whole prostate salvage HIFU can be avoided in the FORECAST study. See FORECAST for more information.