
Pre-operatively Predict The LARS Score
POLARS, or the Pre-Operative Low Anterior Resection Syndrome Score, is a mathematical model, accessed by the internet that has been designed to help patients and doctors understand the risk of poor bowel function after rectal cancer surgery. You can see a short video here and a pdf document here. There is also a longer video presentation here with accompanying audio.
The interactive POLARS tool is easy to use. Simply input an individual’s data for the variables shown below and press calculate.
The POLARS webpage does not collect or store any personal information.
| Please enter the following: | |
| Age at surgery: | |
| Gender: |
|
| TME / PME: |
|
| Tumour height (cms): | |
| Stoma? |
|
| Pre-op radiotherapy: |
|
| Results: | |
| LARS score: | … |
How should I interpret the POLARS score?
POLARS, the pre-operative LARS score, is presented as a single value Battersby et al, (1). It is a prediction of the symptoms reported according to a bowel function questionnaire called LARS (2). The cut off values of 0 to 20 (no LARS), 21 to 29 (minor LARS), and 30 to 42 (major LARS) are taken from the original development and validation of the Low Anterior Resection Syndrome score paper, Emmertsen et al, Aarhus, Denmark
The LARS questions are shown below and the points allocated are shown in red.
Please note that the predicted score is a single value based on the ‘average’ person. However the affect of bowel dysfunction on quality of life is highly individual. We have described this previously (3) and we have developed a, traffic light based, people diagram to try to represent scenario based variations in bowel related quality of life, as displayed below.
The LARS Questionnaire and Scoring Summary
The aim of this questionnaire is to assess your bowel function. Please tick only one box for each question. It may be difficult to select only one answer, as we know that for some patients symptoms vary from day to day. We would kindly ask you to choose one answer which best describes your daily life. If you have recently had an infection affecting your bowel function, please do not take this into account and focus on answering questions to reflect your usual daily bowel function.
Q.1 : Do you ever have occasions when you cannot control your flatus (wind)?
| □ | No, never | 0 |
| □ | Yes, less than once per week | 4 |
| □ | Yes, at least once per week | 7 |
Q.2 : Do you ever have any accidental leakage of liquid stool?
| □ | No, never | 0 |
| □ | Yes, less than once per week | 3 |
| □ | Yes, at least once per week | 3 |
Q.3 : How often do you open your bowels?
| □ | More than 7 times per day (24 hours) | 4 |
| □ | 4-7 times per day (24 hours) | 2 |
| □ | 1-3 times per day (24 hours) | 0 |
| □ | Less than once per day (24 hours) | 5 |
Q.4 : Do you ever have to open your bowels again within one hour of the last bowel opening?
| □ | No, never | 0 |
| □ | Yes, less than once per week | 9 |
| □ | Yes, at least once per week | 11 |
Q.5 : Do you ever have such a strong urge to open your bowels that you have to rush to the toilet?
| □ | No, never | 0 |
| □ | Yes, less than once per week | 11 |
| □ | Yes, at least once per week | 16 |
The allocated points per question are indicated in the right hand column, the score from each of the five answers is added together to give a final score between 0-42.
Interpretation:
0-20 = No LARS 21-29 = Minor LARS 30-42 = Major LARS
A consent aid that highlights the risk of impaired bowel-related quality of life (BQoL) after a restorative rectal cancer resection.
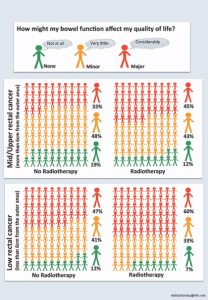
The 2 key risk factors are preoperative radiotherapy and tumor height ≤6 cm from the anal verge. BQoL is trichotomized into major, minor, or no impairment. The predicted percentages are derived from the multifactorial regression model (3).